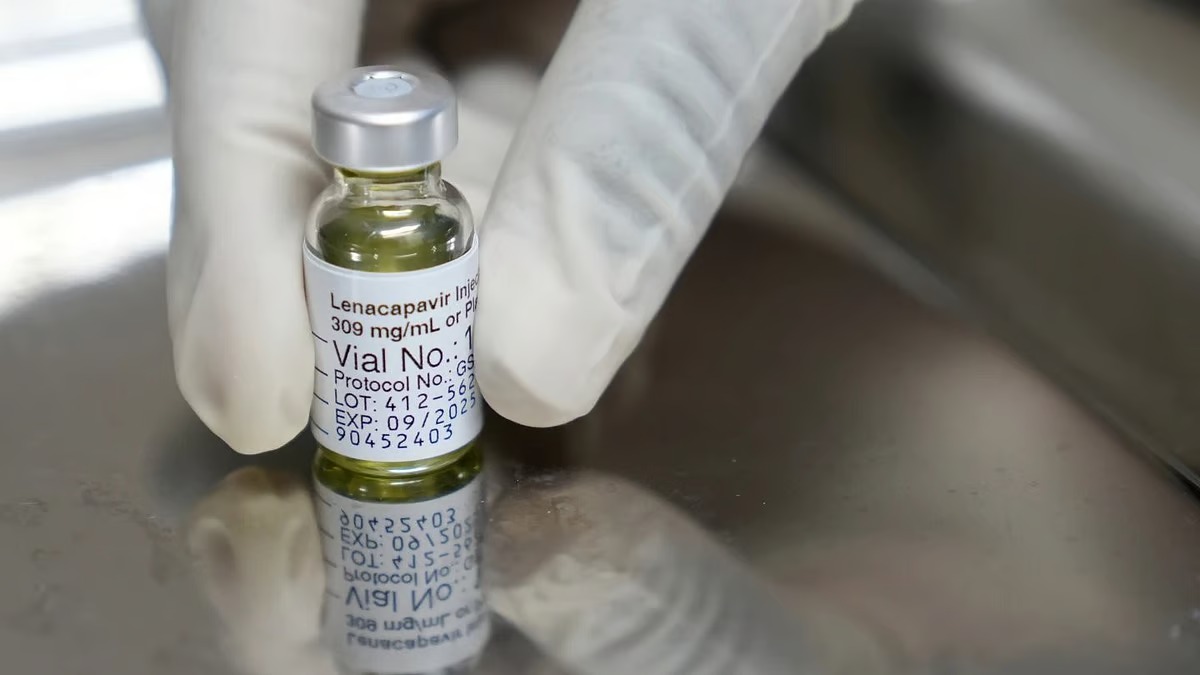
Gilead Sciences, a well-known pharmaceutical company, has developed a new medicine called lenacapavir, which is being reviewed under the priority approval process by the U.S. Food and Drug Administration (FDA). If approved, this drug could revolutionize HIV prevention by offering a long-acting, twice-yearly injectable alternative to daily oral HIV prevention medicines.
What is Lenacapavir?
Lenacapavir is a capsid inhibitor, meaning it targets a key part of the HIV virus to prevent infection and replication. This drug is already available under the brand name Sunlenca for treating patients with multidrug-resistant HIV in the United States, Europe, and other countries.
Now, Gilead is seeking FDA approval for its use in pre-exposure prophylaxis (PrEP)—a preventive treatment for people at risk of contracting HIV.
How is Lenacapavir Different from Existing PrEP Options?
Current HIV prevention (PrEP) medications, such as Truvada, must be taken once a day. While effective, many people find it hard to remember to take a daily pill, which can lower the medicine’s effectiveness.
Lenacapavir offers a new approach—it is an injectable medication that only needs to be taken twice a year. This long-lasting protection could make it easier for people to stay on the medication and lower HIV infection rates worldwide.
How Effective is Lenacapavir?
Clinical trials have shown impressive results:
- In the PURPOSE 1 trial, not a single person taking lenacapavir got HIV, which means the drug completely prevented infections.
- In the PURPOSE 2 trial, the medicine showed a 96% reduction in HIV risk compared to those not using it.
- These results show that lenacapavir is even more effective than existing oral PrEP medications.
Safety and Side Effects
Lenacapavir was generally found to be safe and well-tolerated in trials. No major safety concerns were reported.
Why is This a Big Deal?
HIV remains a global health crisis, with millions of people at risk of infection. In 2023, about:
- 2.6 million people in Africa used PrEP.
- 491,201 people in the U.S. were on PrEP.
- 263,725 people in the top five European countries (France, Germany, Italy, Spain, and the UK) used PrEP.
These numbers are expected to rise by 30–40% over the next decade, showing the urgent need for better, more convenient prevention options.
Lenacapavir’s twice-yearly injection could increase adherence, making it easier for people to stay protected against HIV. This could be especially useful in regions with high infection rates and low access to healthcare, such as sub-Saharan Africa.
Challenges and Concerns
While the medical community is excited about lenacapavir, some experts have raised concerns:
- Cost: Will it be affordable for people in low-income countries?
- Long-term effectiveness: How well will it work over many years?
- Resistance: Could the HIV virus develop resistance to this new drug?
What Happens Next?
The FDA will announce its decision on June 19, 2025. If approved, lenacapavir will become the first and only twice-yearly injectable PrEP available for HIV prevention.
Gilead has also submitted applications to the European Medicines Agency to make lenacapavir available in Europe and low-income countries.
Conclusion
Lenacapavir may prove to be a game-changer in the battle against HIV, providing an easier, more effective alternative to once-daily PrEP pills. If approved, it potentially has the ability to prevent thousands of new infections annually, making it easier and more convenient for people around the world to prevent HIV.
Also Read: Vaccine Stocks Surge on HKU5 CoV-2 Bat Virus Discovery